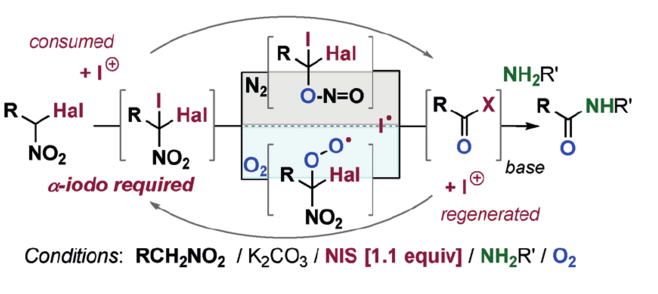
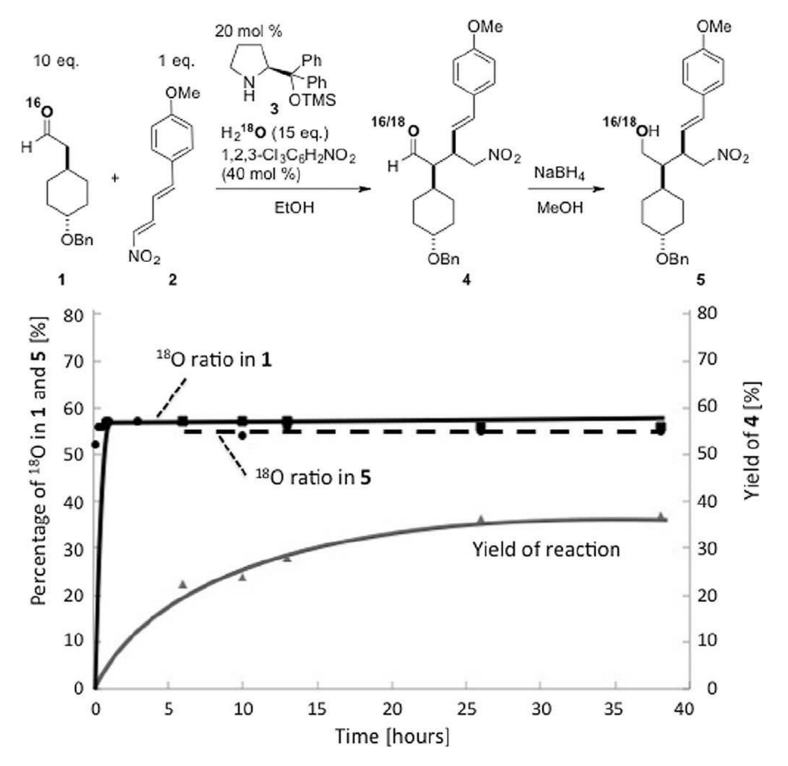
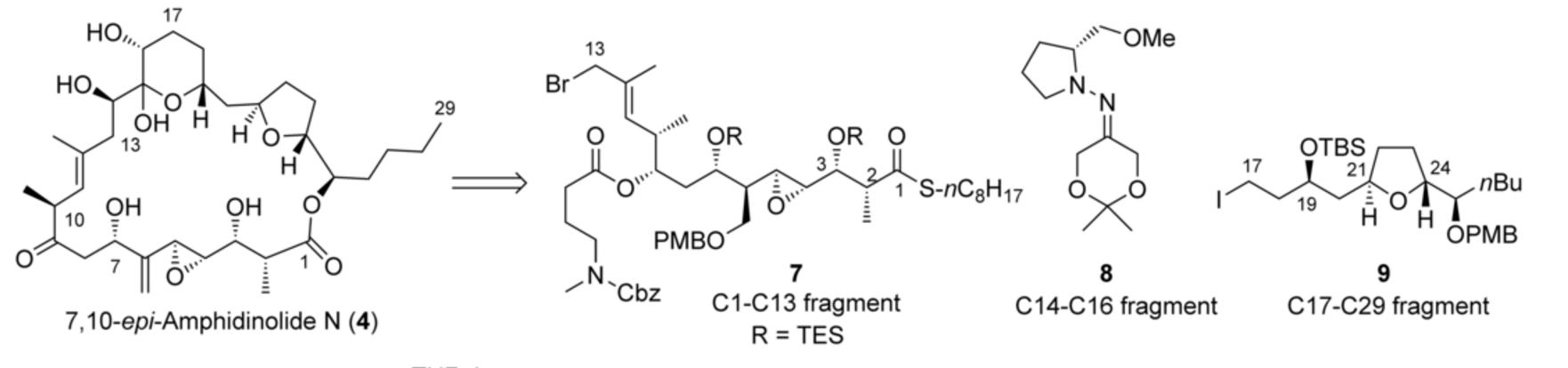
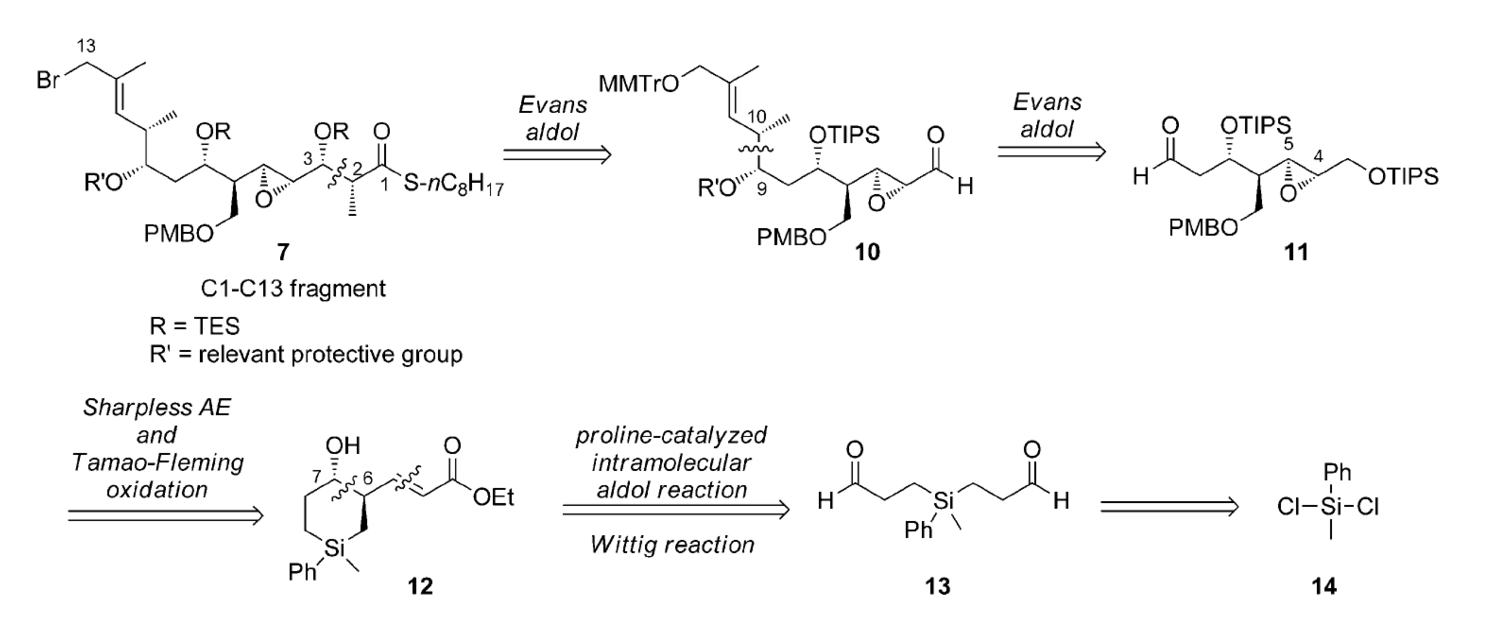
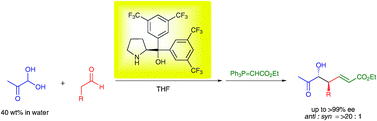
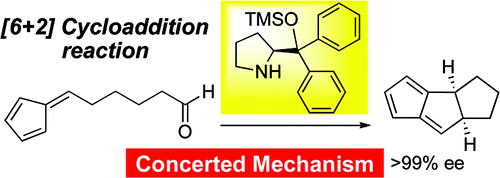
Prof. hayashi received "Humboldt Research Award", and he was invited to the Humboldt Fundation's award ceremony in Bamberg, Germany, on March 28.
【Humboldt Research Award】
This award was established by the Alexander von Humboldt Fundation, the
German government's international organization for academic activities.
This award is given to internationally renowned researchers who have made
significant achievements that will remain important for the future and
who are expected to continue to work at the forefront of scholarship.
Reason for Award
Professor Hayashi has been one of the major leaders in the field of asymmetric
organocatalysis since the early 2000s. One of his most importnat achievements
is the development of a highly versatile silyl ether-type catalyst in 2005.
This catalyst is currently the most frequently and academic natural product
synthesis as well as in various reactions in the pharmaceutical industry.

Professor Hayashi
Photo credit:Humboldt Foundation [Claus Riegel]

Award Ceremony
Photo credit:Humboldt Foundation [Claus Riegel]

During the interval of the ceremony, each movement of Haydon's "String
Quartet in E-flat major, op. 33, No. 2" was performed.
Photo credit:Humboldt Foundation [Claus Riegel]
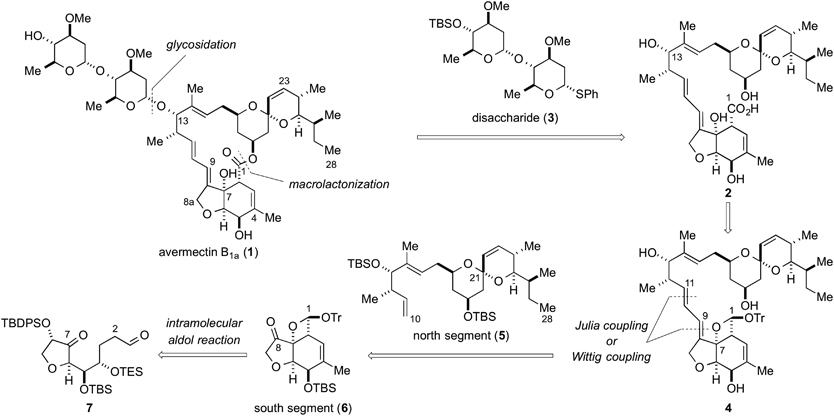
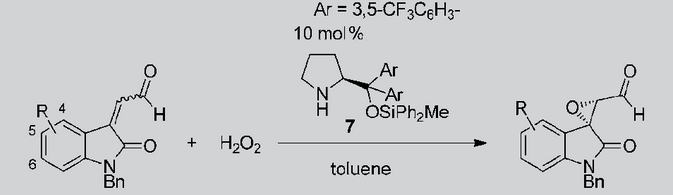
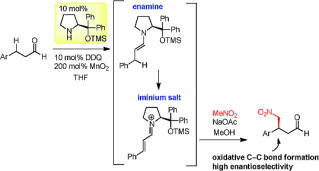
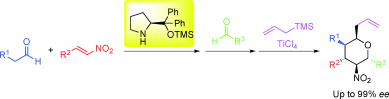
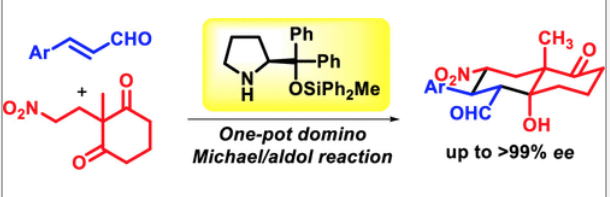
Asymmetric Synthesis of Noradamantane Scaffolds via Diphenylprolinol Silyl Ether-Mediated Domino Michael/Epimerization/Michael (or Aldol)/1,2-Addition Reactions
K. Daskalakis, N. Umekubo, S. Indu, G. Kawauchi, T. Taniguchi, K, Monde,
Y. Hayashi
Angew. Chem. Int. Ed. 2025, e202500378
https://doi.org/10.1002/anie.202500378
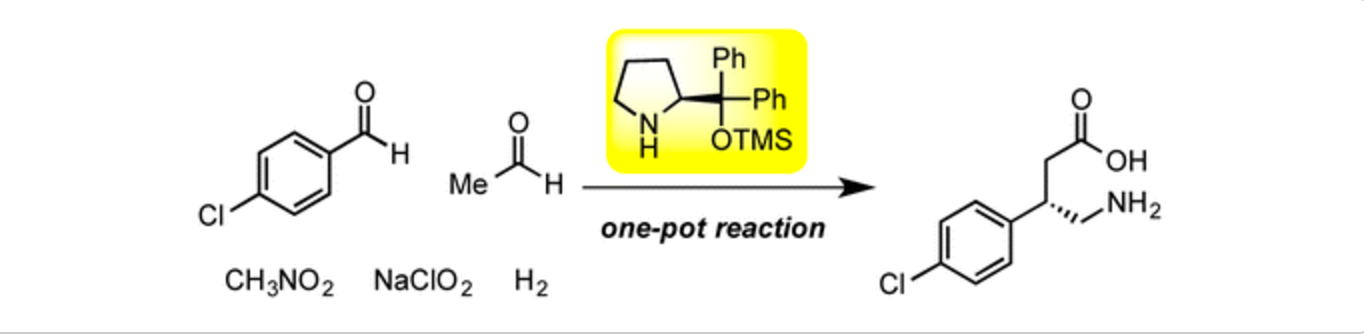
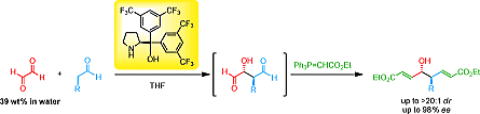
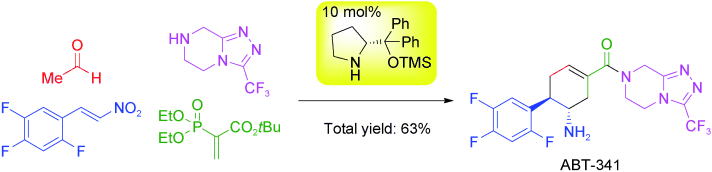
Enantioselective Synthesis of α-Trifluoromethyl tert-Alcohols and Amines
via Organocatalyst-Mediated Aldol and Mannich Reactions
Y. Hayashi, H. Odaira, M. Tomikawa, N. Mori, T. Taniguchi, K. Monde
Asian J. Org. Chem. 2025, e202500035
https://aces.onlinelibrary.wiley.com/doi/10.1002/ajoc.202500035
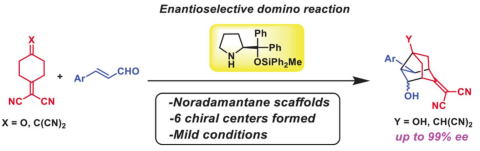
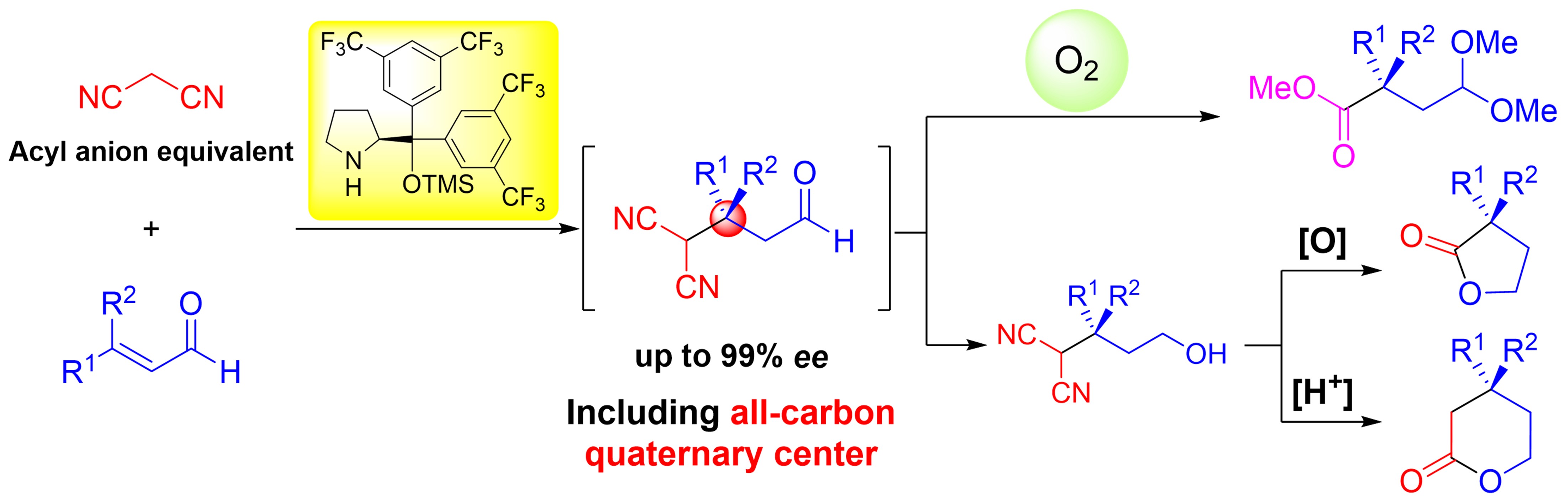
Synthesis of Consecutive All-carbon Quaternary Centers via Three-Step reactions
X-L. han, H. Ban, N. Mori, Y. Hayashi
Org. lett. 2024, 26, 10840-10845
https://doi.org/10.1021/acs.orglett.4c03945
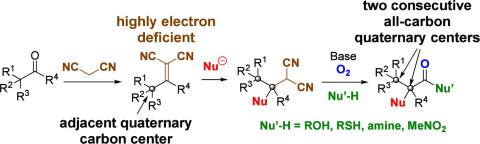
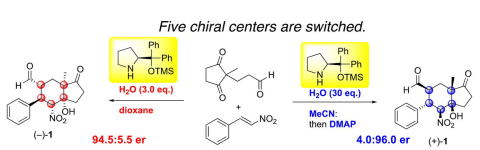
Switch of Five Contiguous Chiral Centers in the Synthesis of Both Enantiomers
of Hajos-Parrish Ketone Analogs via Diphenylprolinol Silyl Ether-Mediated
Domino Reaction
Y. Hayashi, Q. Xu, S. Koshino
Chem. Eur. J., 2024, e202403580
https://doi.org/10.1002/chem.202403580
Title: 実用的有機触媒の開発と環境調和型合成プロセスの開発 (Development of Practical Organocatalysts
and Environmentally Conscious Synthetic processes)
URL: https://www.sgkz.or.jp/prize/science/56/

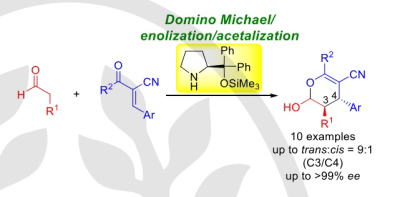
Enantioselective Synthesis of Substituted Dihydropyrans by Organocatalyst-Mediated
Domino Michael/enolization/acetalization Reactions
Y. Hayashi, X.-L. Han, W. R. Hack
Synlett, 2024, accepted
https://www.thieme-connect.com/products/ejournals/pdf/10.1055/a-2324-8899.pdf
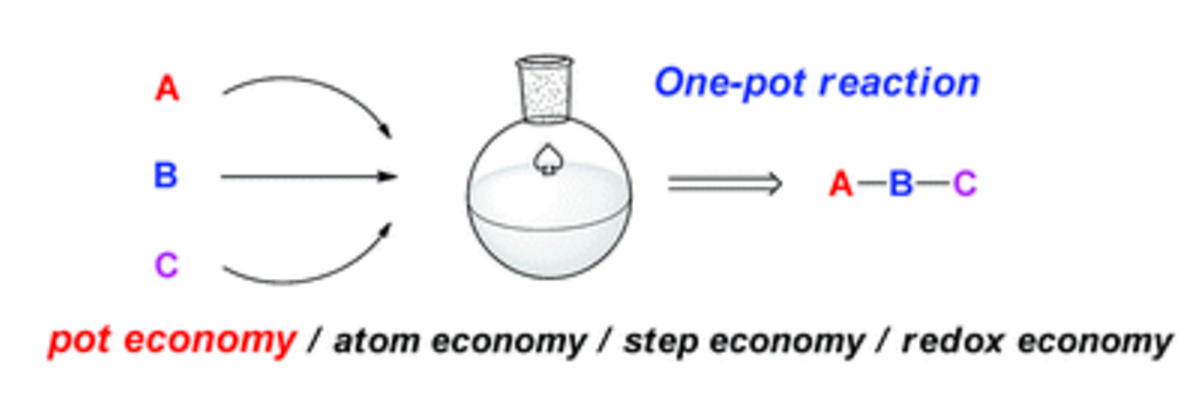
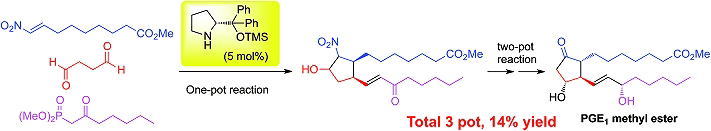
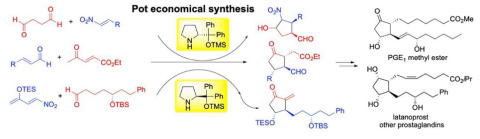
Pot-economical total synthesis of prostaglandins via organocatalyst-mediated asymmetric reactions
Yujiro Hayashi
Bull. Chem. Soc. Jpn., 2024, 97, uoea039
https://doi.org/10.1093/bulcsj/uoae039
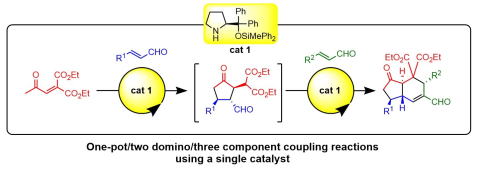
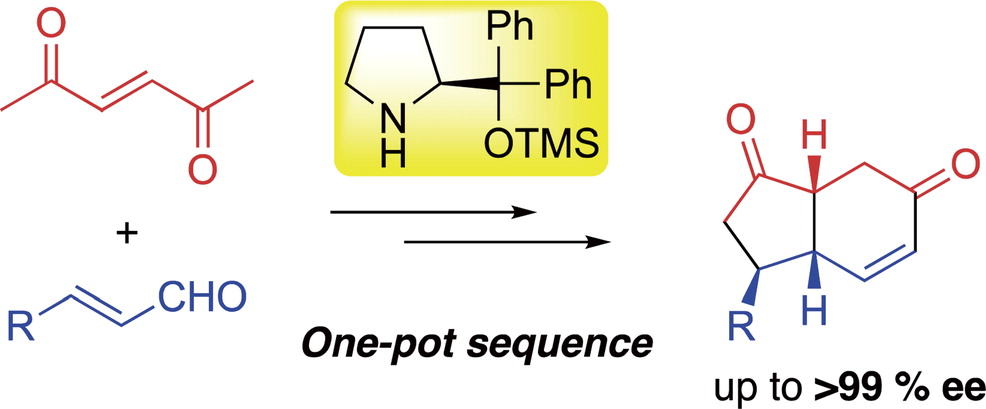
Organocatalyst-mediated asymmetric one-pot/two domino/three-component coupling
reactions for the synthesis of trans-hydrindanes
N. Mori, T Tachibana, N. Umekubo, Y. Hayashi*
Chem. Sci., 2024, 15, 5627-5632
DOI: 10.1039/d4sc00193a
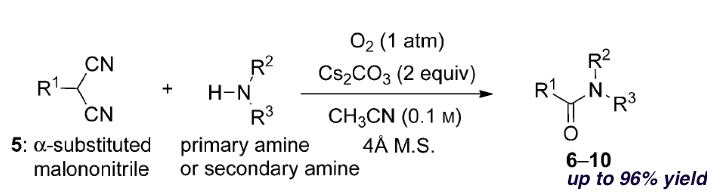
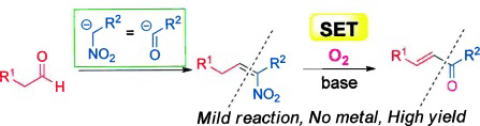
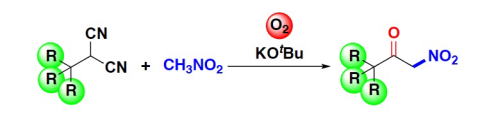
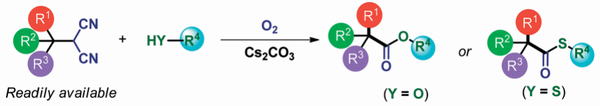
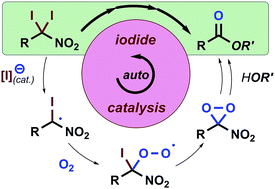
Oxidative Synthesis of α-Nitroketones from α-Substituted Malononitrile
and Nitrmethane Using Molecular Oxygen without Condensation Reagents
Y. Hayashi, E. Cocco, H. Odaira, H. Matoba, N. Mori
Eur. J. Org. Chem. 2023.ASAP e202300964
doi/10.1002/ejoc.202300964
https://www.organic-chemistry.org/Highlights/2023/13November.shtm
https://iupac.org/event/international-conference-on-organic-synthesis-23-icos/
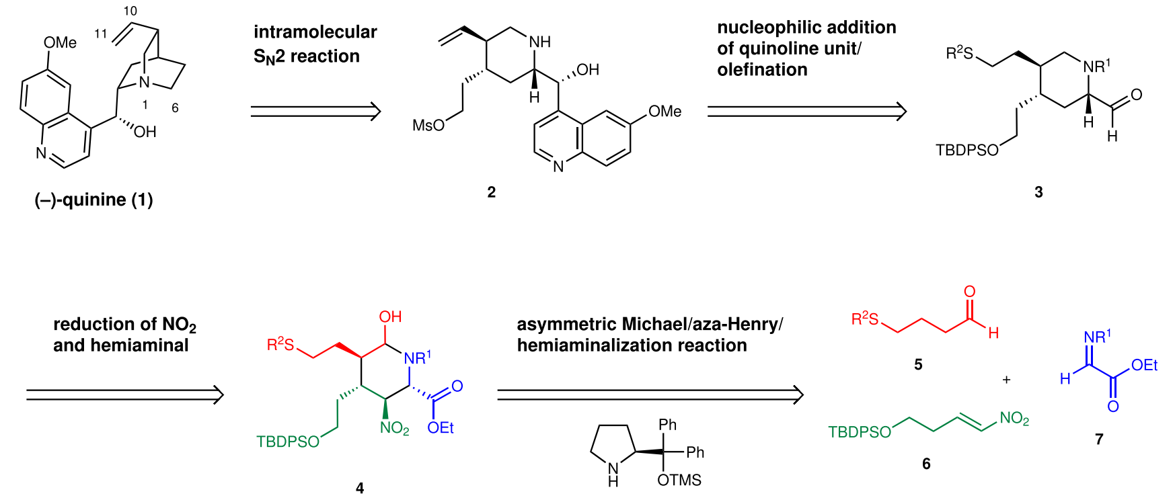
Organocatalyst Mediated Pot-Economical Total Synthesis of (-)-Quinine and its Derivatives
T. Terunuma, G. Kawauchi, Y. Hayashi*
Asian. J. Org. Chem. 2023, ASAP e202300256
DOI.org/10.1002/ajoc.202300256
Invited paper for the special issue dedicated to Prof. Keiji Maruoka on
the occasion of his 70th birthday
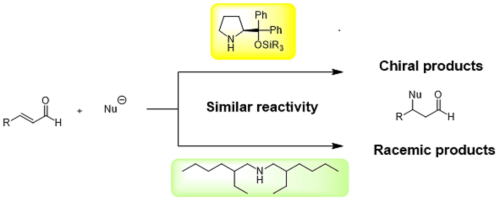
Bis(2-ethylhexyl)amine as an Effective Organocatalyst for the Racemic Reactions
of α,β-Unsaturated Aldehydes Involving an Iminium Ion
Y. Hayashi*, X.-L. Han, N. Mori
Synlett . 2023, ASAP
DOI: 10.1055/a-2179-5916
Invited paper for the special issue dedicated to prof. Hisashi Yamamoto
on the occasion of his 80th birthday
Organocatalyst-mediated, pot-economical total synthesis of latanoprost
G. Kawauchi, Y. Suga, S. Toda, Y. Hayashi*
Chem. Sci. 2023, ASAP.
DOI:org/10.1039/D3SC02978F
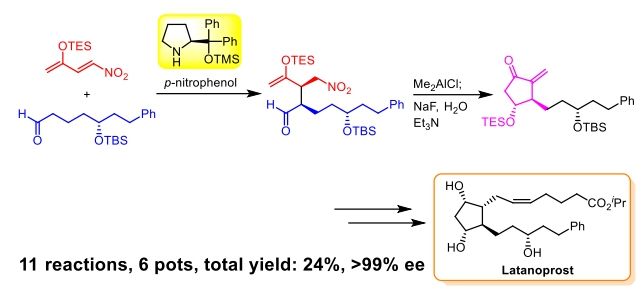
This paper was selected as a cover picture!!

Secondary Amine-Mediated Domino Reaction for the Synthesis of
Substituted Quinolines from Dicyanoalkenes and Eneynals
Y. Hayashi*, X.-L. Han, N. Mori
Chem. Eur. J., ASAP.
https://doi.org/10.1002/chem.202301093
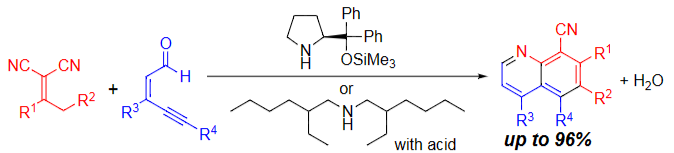
https://chematels.com/article/clfm2318n20pv0bzv1xy1elzv

DOI:https://doi.org/10.1038/s41467-022-34916-z
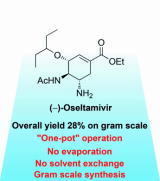
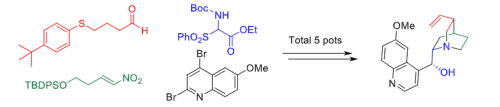
Organocatalyst-mediated five-pot synthesis of (-)-quinine
T. Terunuma, Y. Hayashi*
Nat. Commun. 2022, 13, 7503.
DOI:https://doi.org/10.1038/s41467-022-34916-z

The press release
Tohoku Univ. HP
https://www.tohoku.ac.jp/japanese/2022/12/press20221208-02-quinine.html
Graduate School of Science and Fuculty of Science, Tohoku University HP
https://www.sci.tohoku.ac.jp/news/20221208-12401.html
Twitter
https://twitter.com/sci_koho/status/1600673681008885760
Cover Picture
DOI: 10.1055/a-1846-5007

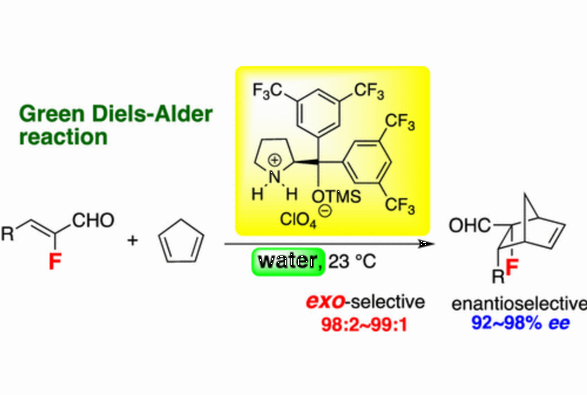
Stereoselective Construction of Fluorinated Quaternary Stereogenic Centers
via an Organocatalytic Asymmetric exo-Selective Diels-Alder Reaction in the Presence of Water
B. P. Bondzic, K. Daskalakis, T. Taniguchi, K. Monde, Y. Hayashi*
Org. Lett. 2022, 24, 7455.
doi.org/10.1021/acs.orglett.2c03043
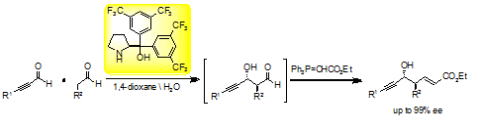
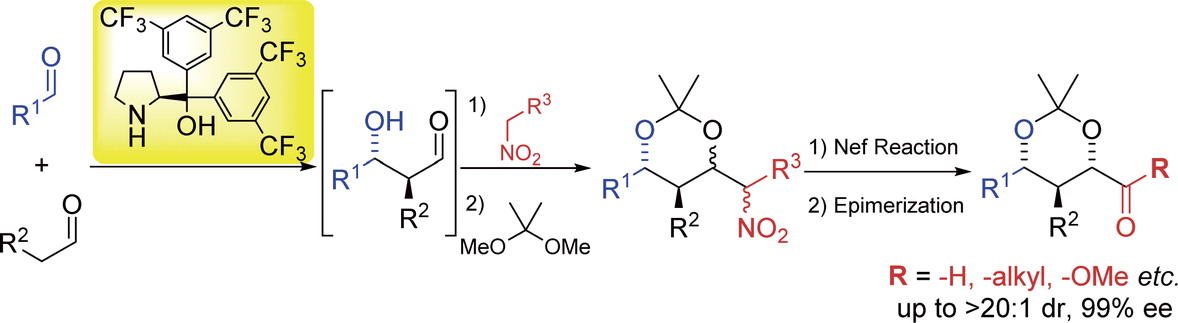
Diarylprolinol as an Effective Organocatalyst in Asymmetric Cross-aldol
Reactions
of Two Different Aldehydes
Y. Hayashi*
Chem. Rec. 2022, e202200159.
DOI:org/10.1002/tcr.202200159

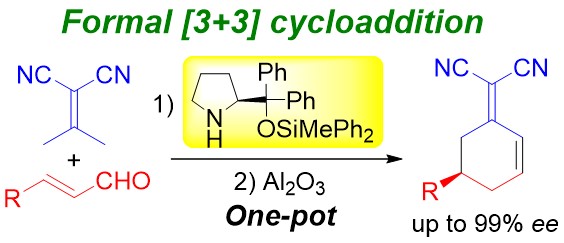
Diphenylprolinol Silyl Ether Catalyzed Asymmetric Formal Carbo [3+3]
Cycloaddition Reaction
of Isopropylidenemalononitrile and α,β-Unsaturated Aldehyde
N. Umekubo, X. Han, N. Mori, Y Hayashi*
Eur. J. Org. Chem. 2022, ASAP.
DOI:org/10.1002/ejoc.202200603
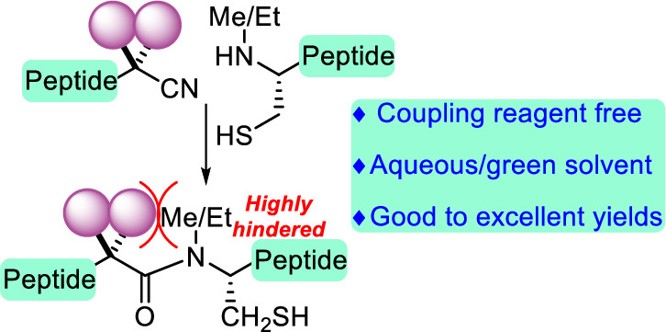
Highly Sterically Hindered Peptide Bond Formation between α,α-Disubstituted α-Amino Acids
and N-Alkyl Cysteines Using α,α-Disubstituted α-Amidonitrile
X. Wang, J. Li, Y. Hayashi*
J. Am. Chem. Soc. 2022, ASAP.
DOI:org/10.1021/jacs.2c02993
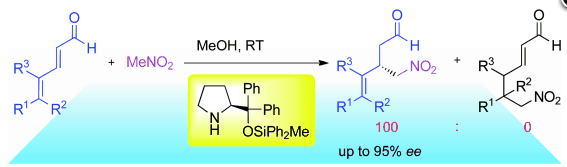
Asymmetric Michael Reaction of Aldehydes and Nitroalkenes
Y. Hayashi*
Org. Synth. 2022, 99, 68.
DOI: 10.15227/orgsyn.099.0068
Asymmetric Michael reaction of malononitrile and
α,β-unsaturated aldehydes catalyzed by diarylprolinol silyl ether
Y. Hayashi*, Y. Hatano, N. Mori
Synlett 2022, ASAP.
DOI: 10.1055/a-1846-5007
Invited cluster paper: Development and application of
novel ligands/catalysts and mechanistic studies on catalysis
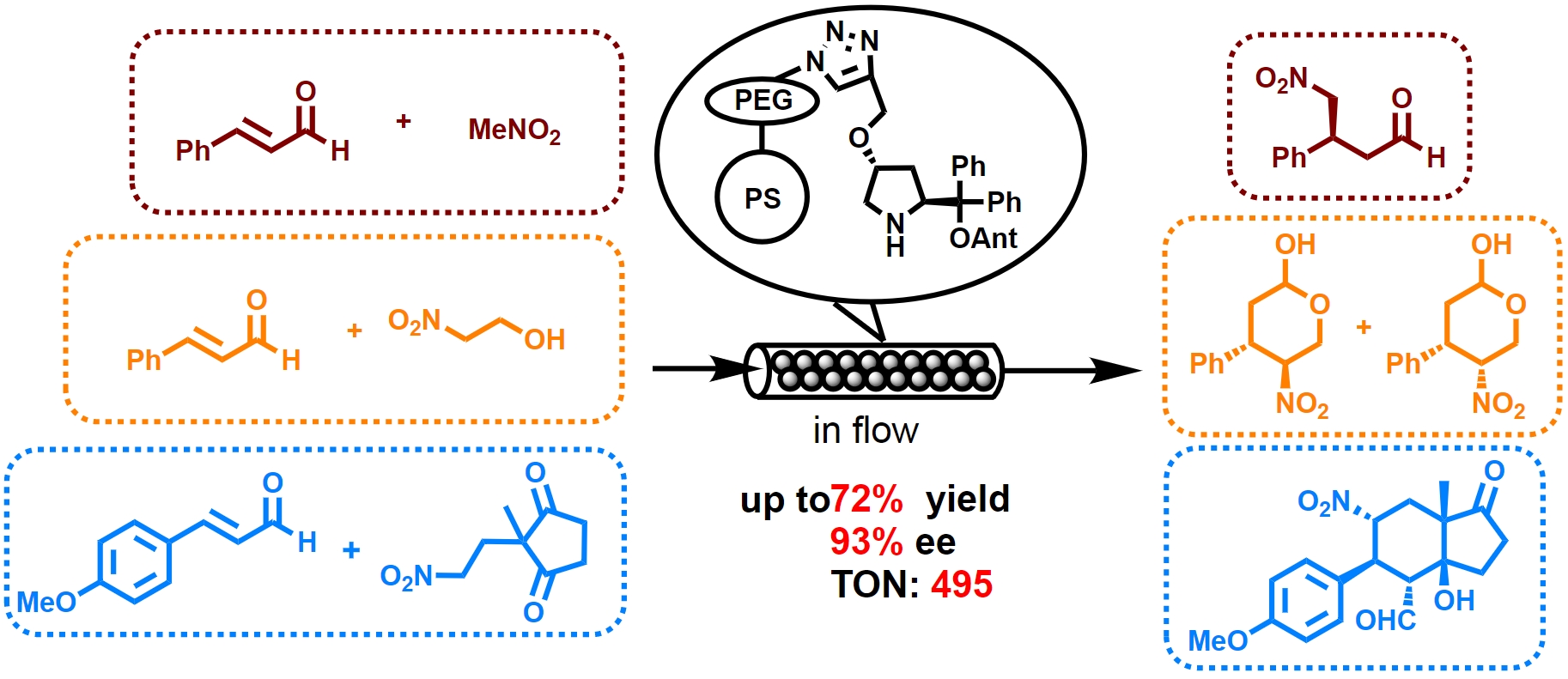
Asymmetric flow reactions catalyzed by immobilized diphenylprolinol alkyl ether:
Michael reaction and domino reactions
Y. Hayashi*, S. Hattori, S. Koshino
Chem Asian J. 2022, ASAP.
DOI:org/10.1002/asia.202200314
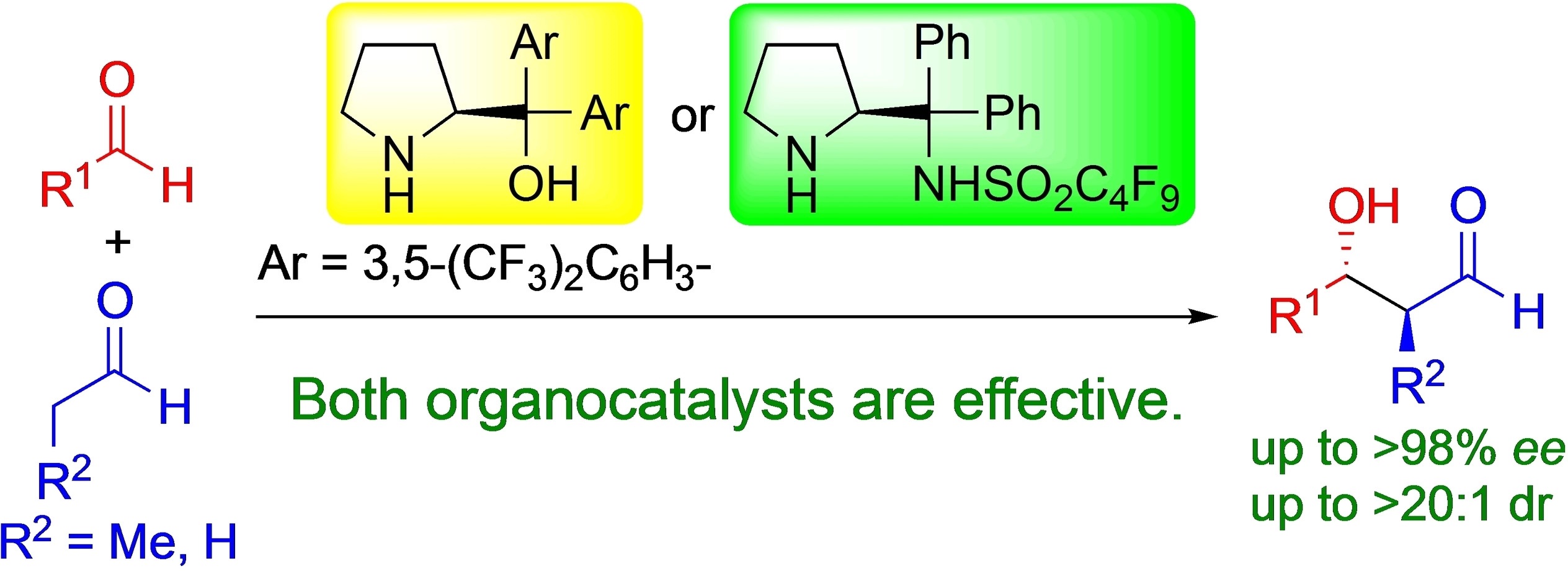
Diarylprolinol with Trifluoromethyl Substituents and Diphenylprolinol-Derived
Perfluoroalkanesulfonamide as Organocatalysts in the Cross-Aldol Reaction
of Aldehydes
Y. Hayashi*, M. Tomikawa, N. Mori
Adv. Synth. Catal. 2022, ASAP.
DOI:org/10.1002/adsc.202200266
Invited paper of the special issue dedicated to Prof. Andreas Pfaltz.
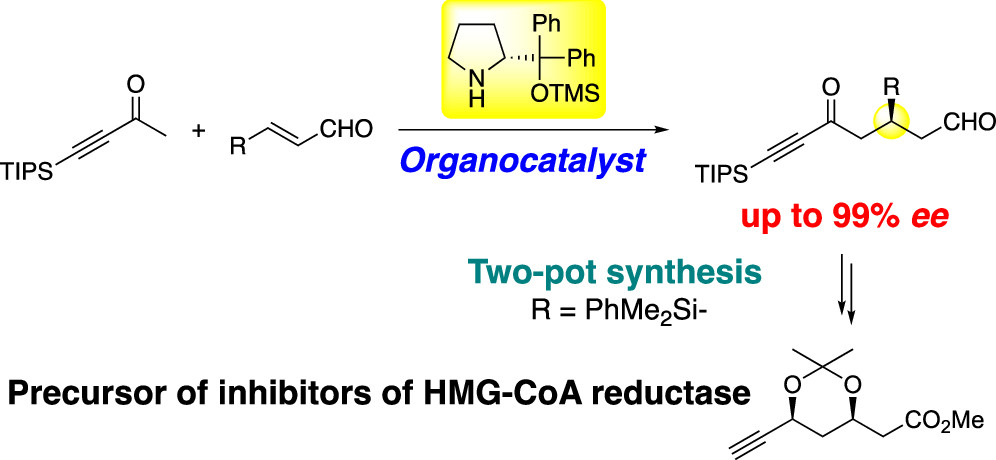
Catalytic asymmetric Michael reaction of methyl alkynyl ketone
catalyzed by diphenylprolinol silyl ether
N. Umekubo, Y. Hayashi*
ACS Org. Inorg. Au, 2022, ASAP.
DOI: org/10.1021/acsorginorgau.1c00054
Asymmetric Synthesis of Pentasubstituted Cyclohexanes
through Diphenylprolinol Silyl Ether Mediated Domino Michael/Michael Reaction
A. S. Odoh, L. Aidanpää, N. Umekubo, H. Matoba, N. Mori, Y. Hayashi*
Eur. J. Org. Chem. 2021, 6670.
DOI: org/10.1002/ejoc.202101106
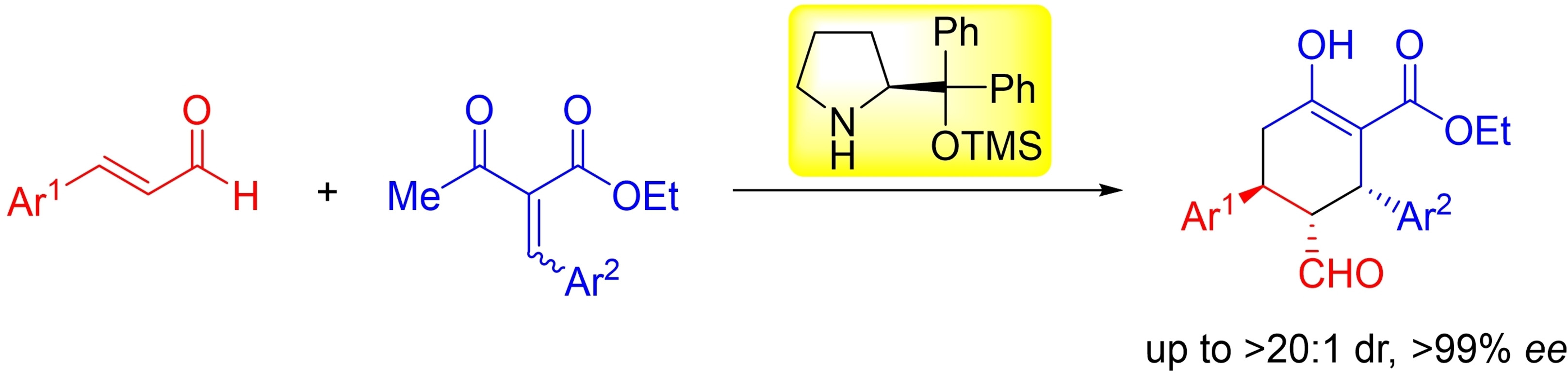
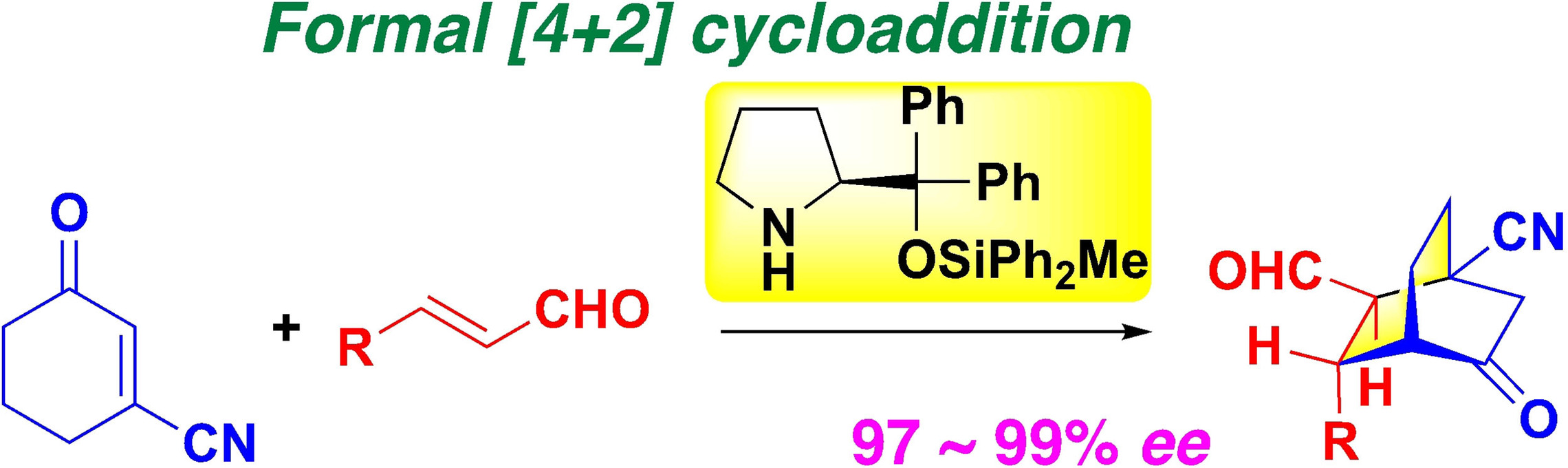
Synthesis of bicyclo[2.2.2]octanes with a quaternary bridgehead carbon
by diphenylprolinol silyl ether mediated domino reaction
N. Umekubo, T. Taniguchi, K. Monde, Y. Hayashi*
Asian. J. Org. Chem. 2021, 10, 3261.
DOI: org/10.1002/ajoc.202100573
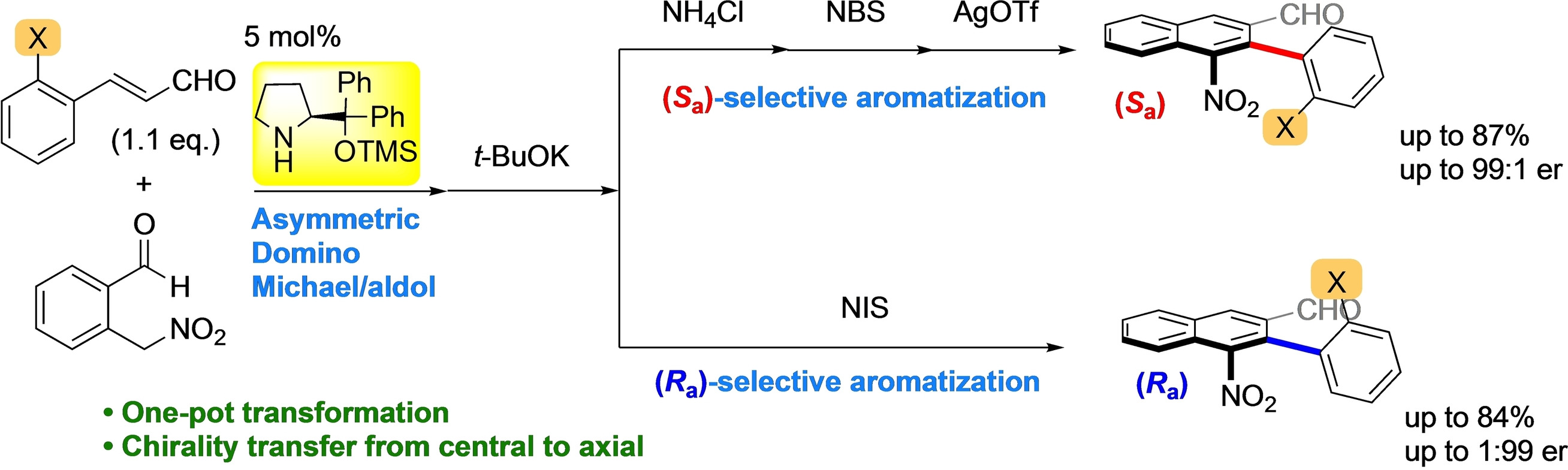
Enantiodivergent one-pot synthesis of axially chiral biaryls using
organocatalyst-mediated
enantioselective domino reaction and central-to-axial chirality conversion
S. Koshino, T. Taniguchi, K. Monde, E. Kwon, Y. Hayashi*
Chem. Eur. J. 2021, 27, 15786.
DOI: org/10.1002/chem.202102797
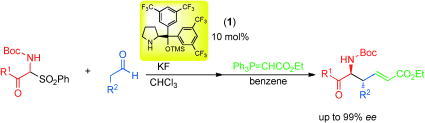
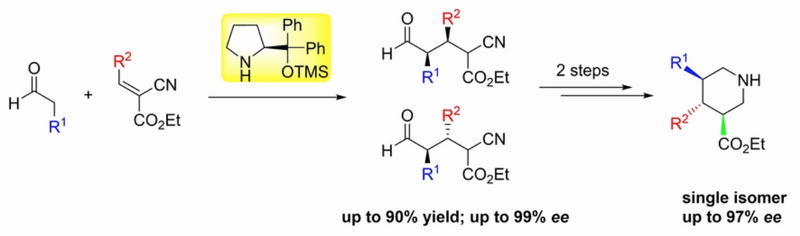
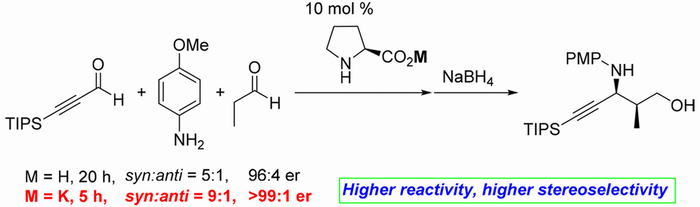
Title: Organocatalyst mediated asymmetric Michael reaction of malononitrile to α,β-unsaturated aldehyde
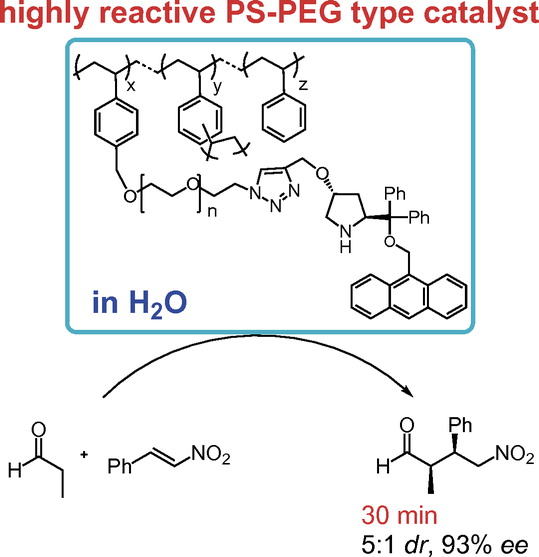
Amphiphilic Immobilized Diphenylprolinol Alkyl Ether Catalyst on PS-PEG Resin
Seitaro Koshino, Shusuke Hattori, Shota Hasegawa, Naoki Haraguchi, Takeshi
Yamamoto,
Michinori Suginome, Yasuhiro Uozumi, and Yujiro Hayashi *
Bull. Chem. Soc. Jpn. 2021, 94, 790.
https://doi.org/10.1246/bcsj.20200355
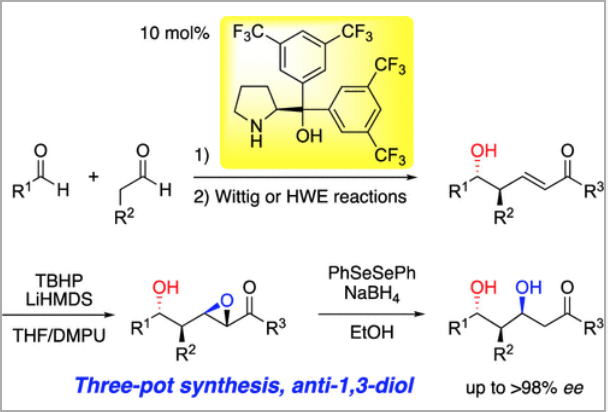
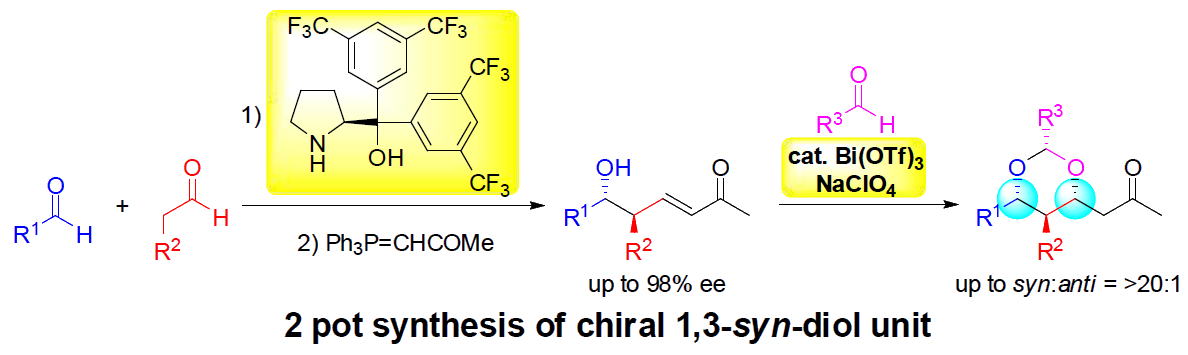
Three-pot synthesis of chiral anti-1,3-diols through asymmetric organocatalytic
aldol
and Wittig reactions followed by epoxidation and reductive opening of the
epoxide
Y. Hayashi*, M. Tomikawa, N. Mori
Org. Lett., 2021, 23, 5896.
DOI: 10.1021/acs.orglett.1c01986
Asymmetric synthesis of functionalized 9-methyldecalins
using a diphenylprolinol silyl ether-mediated domino Michael/Aldol reaction
Y. Hayashi*, H. A. Salazar, S. Koshino
Org. Lett., 2021, 23, 6654.
DOI: 10.1021/acs.orglett.1c02196
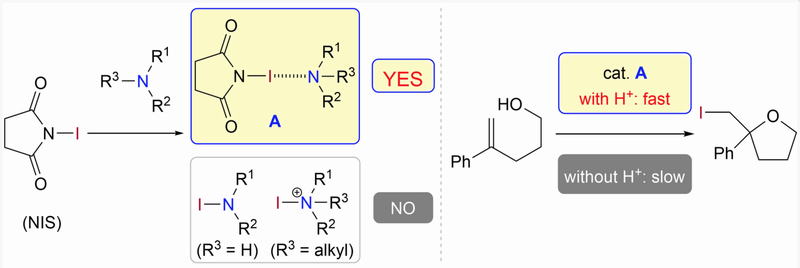
Halogen Bonding of N-Halosuccinimides with Amines
and Effects of Brønsted Acids in Quinuclidine-Catalyzed Halocyclizations
J. Li, E. Kwon, M. J. Lear, Y. Hayashi*
Helv. Chim. Acta., 2021, 104, ASAP.
DOI: org/10.1002/hlca.202100080
Invited paper of the special issue dedicated to the 75th birthday of Prof. E. P. Kundig.
Title: ''Pot-Economical Synthesis of Prostaglandins''
Oxidative peptide bond formation of glycine–amino acid
using 2-(aminomethyl)malononitrile as a glycine unit
Xiaoling Wang, Jing Li and Yujiro Hayashi*
Chem. Commun., 2021, 57, 4283.
DOI: 10.1039/D1CC00130B
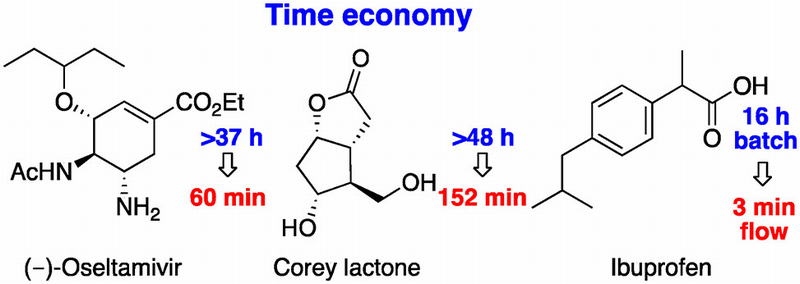
![]()
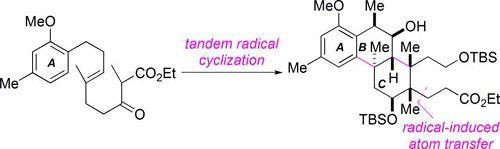
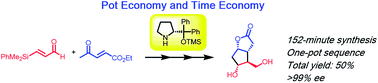
Time Economy in Total Synthesis
Yujiro Hayashi*
J. Org. Chem. 2021, 86, 1.
This article was selected as cover picture.
https://dx.doi.org/10.1021/acs.joc.0c01581

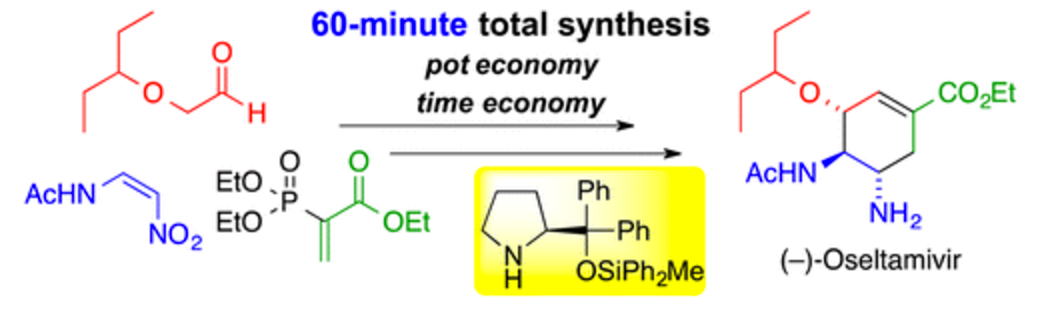
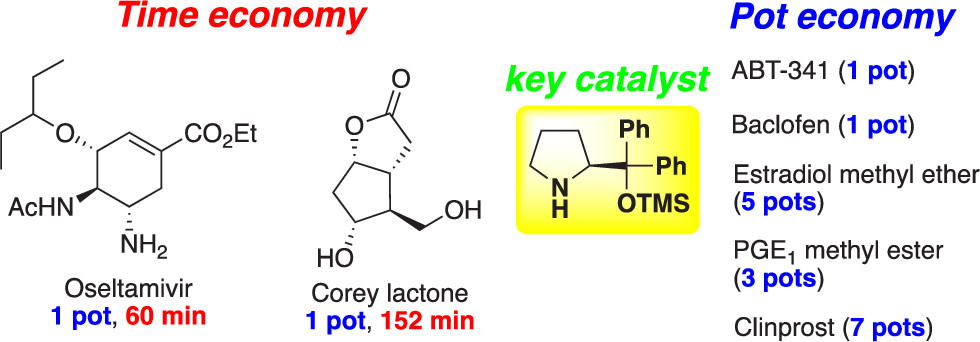
Time and Pot Economy in Total Synthesis
Yujiro Hayashi
Acc. Chem. Res. 2021, 54, 1385.
https://doi.org/10.1021/acs.accounts.0c00803
Amphiphilic Immobilized Diphenylprolinol Alkyl Ether Catalyst on PS-PEG Resin
Seitaro Koshino, Shusuke Hattori, Shota Hasegawa, Naoki Haraguchi, Takeshi
Yamamoto,
Michinori Suginome, Yasuhiro Uozumi, and Yujiro Hayashi *
Bull. Chem. Soc. Jpn. 2021, 94, 790.
https://doi.org/10.1246/bcsj.20200355
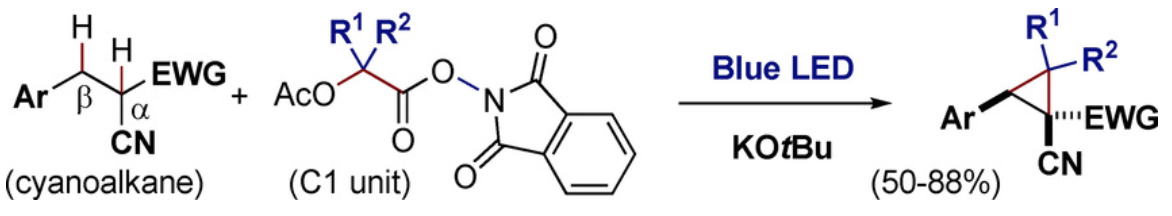
Direct Cyclopropanation of α‐Cyano β‐Aryl Alkanes by Light‐Mediated
Single Electron Transfer Between Donor–Acceptor Pairs
Jing Li, Martin J. Lear, Yujiro Hayashi*
Chem. Eur. J. 2021, 27, 5901.
https://doi.org/10.1002/chem.202100341
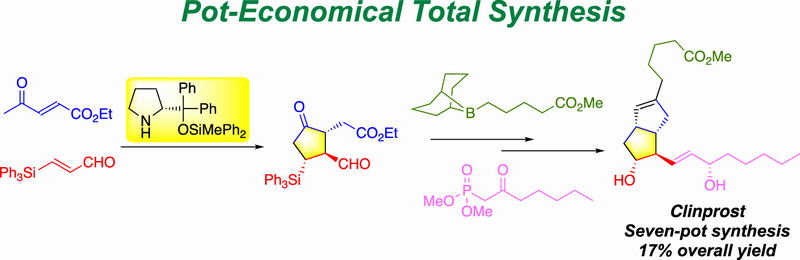
Pot-Economical Total Synthesis of Clinprost
Nariyoshi Umekubo and Yujiro Hayashi*
Org. Lett. 2020, 22, 9365.
https://doi.org/10.1021/acs.orglett.0c03616
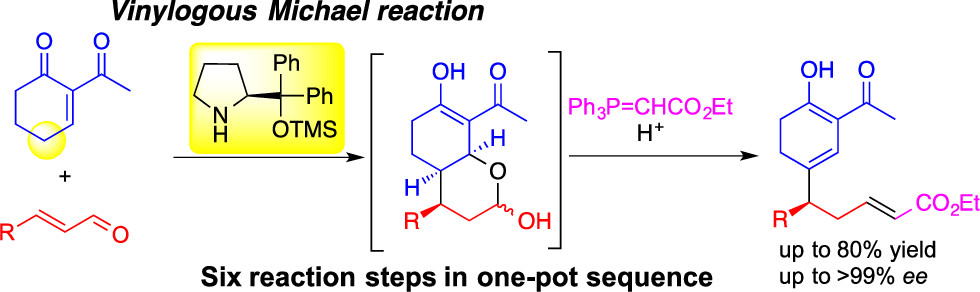
Asymmetric Domino Reaction of α,β-Unsaturated Aldehydes and α-Acyl α,β-Unsaturated
Cyclic Ketones Catalyzed by Diphenylprolinol Silyl Ether
Yujiro Hayashi*, Yurina Suga, and Nariyoshi Umekubo
Org. Lett. 2020, 22, 8603-8607
https://doi.org/10.1021/acs.orglett.0c03190
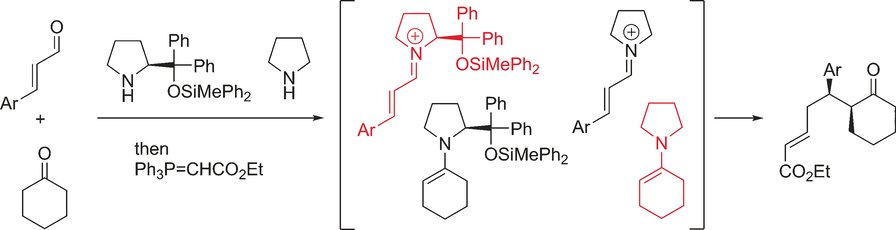
Evidence for an enolate mechanism in the
asymmetric Michael reaction of a,b-unsaturated
aldehydes and ketones via a hybrid system of two
secondary amine catalysts
Nariyoshi Umekubo, Takahiro Terunuma, Eunsang Kwon and Yujiro Hayashi
Chem. Sci., 2020, 11, 11293-11297
https://doi.org/10.1039/D0SC03359F
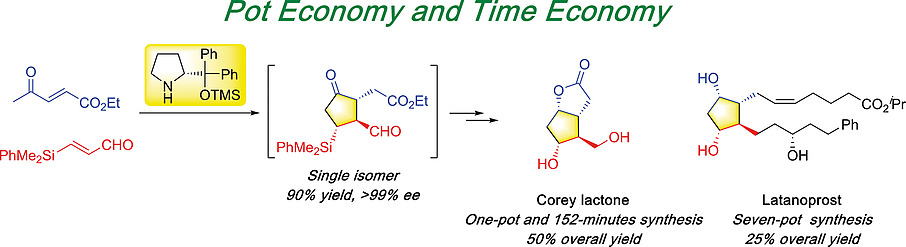
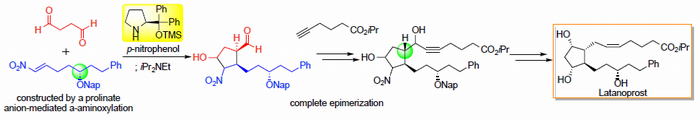
Asymmetric synthesis of Corey lactone and latanoprost
Nariyoshi Umekubo and Yujiro Hayashi
Eur. J. Org. Chem. 2020, 39, 6221.
https://doi.org/10.1002/ejoc.202001063

Inversion of the axial information during oxidative aromatization in the
synthesis of axially chiral biaryls using organocatalyst as a key step
Seitaro Koshino, Akira Takikawa, Keiichi Ishida, Tohru Taniguchi, Kenji Monde, Eunsang Kwon, Shigenobu Umemiya, Yujiro Hayashi
Chem. Eur. J. 2020, 26, 4524-4530
10.1002/chem.201905814

Cover Profile
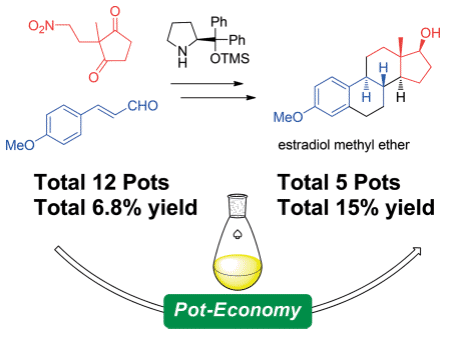
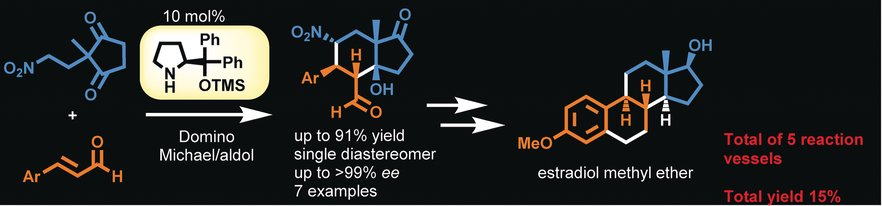
Total Synthesis of Estradiol Methyl Ether and Its Five‐Pot Synthesis with an Organocatalyst
Seitaro Koshino, Eunsang Kwon, Yujiro Hayashi
Eur. J. Org. Chem., 2018, 41, 5629-5638
DOI: 10.1002/ejoc.201800910

Asymmetric synthesis of biaryl atropisomers using an organocatalyst‐mediated
domino reaction as a key step
Y. Hayashi, A. Takikawa, S. Koshino, K. Ishida, Chem. Eur. J. 2019, 25, 10319–10322.
https://doi.org/10.1002/chem.201902767





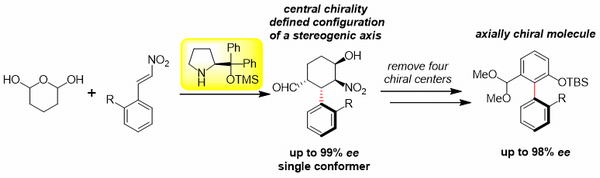
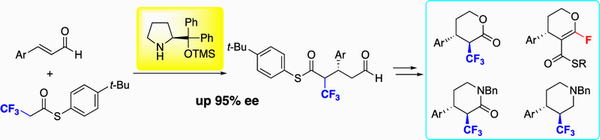

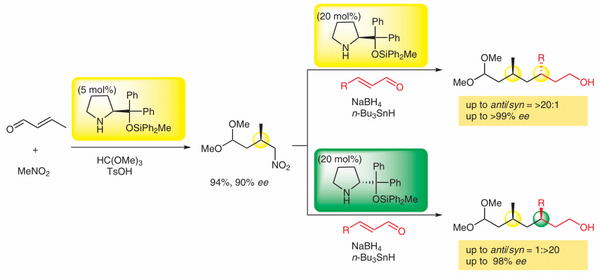



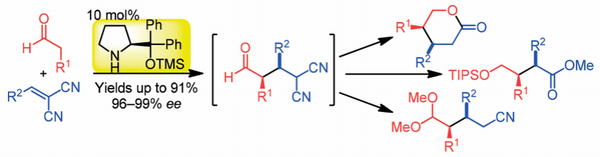




http://www.sci.tohoku.ac.jp/mediaoffice/20180313-9565.html
see also invitation lectures.

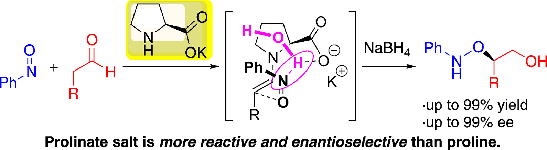
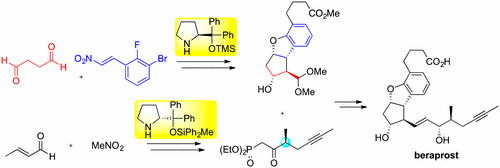

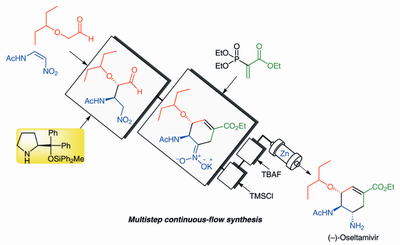




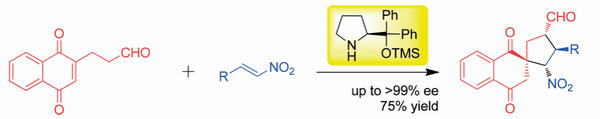
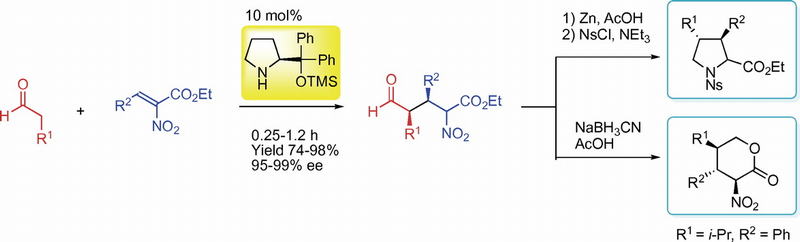
Org. Lett.,18, 3426-3429(DOI : 10.1021/acs.orglett.6b01595)
Yujiro Hayashi and Shin Ogasawara
Reviewed in chemistry world
introduced in Chemical Engineering News